Potassium Dichromate Colour Change
You should check the result as soon as the potassium dichromateVI solution turns green - if you leave it too long the Schiffs reagent might start to change colour in the secondary alcohol case as well. When the solution of K 2 Cr 2 O 7 reacts with an alkali ionic salt a yellow solution is obtained because of the potassium.

Use Of Acidified Potassium Dichromate To Distinguish Between Alehydes And Ketones Youtube
In this glycerol and dichromate ions react to give a brown colour to the solution.
. This type of titration majorly depends on the track change in pH or a pH meter. The oxidation of the alcohol to an aldehyde is indicated by the colour change of the dichromate solution as it is reduced from the orange colour of Cr 2 O 7 2 to the green of chromiumIII ions Cr 3. Anodizing is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts.
Chemical Properties of Potassium Dichromate. These change colour in the presence of an oxidising agent. This is often seen in redox titrations.
Iii Change in state of substance. Chemical Oxygen Demand or COD is a measurement of the oxygen required to oxidize soluble and particulate organic matter in water. Then the chromic ions oxidize the glycerol and reduce into chromous ions by giving a blue colour to the solution in the presence of nitric acid.
Enter the email address you signed up with and well email you a reset link. Ionic radii colour of ions catalytic property magnetic properties interstitial compounds. The sodium dichromate is filtered out and reduced with carbon to produce chromiumIII oxide sodium carbonateand carbon monoxide.
Unlike permanganate dichromate titrations require an indicator. It is a good idea to first carry out a rough titration in order to become familiar with the colour change at the end point. There are several such indicators - such as diphenylamine sulphonate.
The mixture is heated gently followed by immediate distillation of the product. TutorMyself Chemistry has already done the notes for you so its just up to you to memorise these and start to test yourself as early as possible. Sodium nitroprusside Complex of Purple colour 3.
Aim To prepare M20 solution of Mohrs salt and. This colour change of orange to yellow and yellow to orange can be explained on the concept of equilibrium of the two ions. Take 2-3 ml of a sample in a test tube.
The chemical reaction between sulphur dioxide gas and acidified potassium dichromate solution is characterized by a change in colour from orange to green. Elimination reaction Saytzeffs rule β. Potassium permanganate potassium dichromate ceric sulphate etc are the common oxidizing agents used in redox titrations.
The sooner you start testing yourself the sooner youll start putting the facts and concepts into your long-term. The combustion reaction of candle wax is characterised by a change in state from solid to liquid and gas because the wax is a solid water formed by the. The Mohr titration is sensitive to the presence of both chloride and bromide ions in solution and.
Butanol is oxidised by sodium dichromate Na 2 Cr 2 O 7 acidified in dilute sulphuric acid to form the aldehyde butanal. COD can be measured in rapidly or in real-time with Real Techs COD instruments to improve wastewater process control and plant efficiency. The solution of potassium dichromate can be directly used for titrations.
4K 2 Cr 2 O 7 4K 2 CrO 4 2Cr 2 O 3 3O 2. TutorMyself Chemistry is all you need to get to grips with the latest Edexcel iGCSE Chemistry Specification 2017. Introducing heat to K 2 Cr 2 O 7 decomposes it into potassium chromate K 2 CrO 4 and produces O 2 gas.
Na 2 SO 3 H 2 SO 4 Na 2 SO 4 H 2 O SO 2 The gas turns potassium dichromate paper acidified with dil. The color of chemicals is a physical property of chemicals that in most cases comes from the excitation of electrons due to an absorption of energy performed by the chemical. K 2 Cr.
There are three indicators that may be used for the titration of Fe 2 with K 2 Cr 2 O 7. The chromiumIII oxide is then reduced to chromium with aluminum metal. With potassium dichromateVI solution you have to use a separate indicator known as a redox indicator.
The sodium chromate is dissolved in water and this solutionis acidified with sulfuric acid to produce the less soluble sodiumdichromate. H 2 SO 4 green. The colour change for all three indicators is green to violet and the standard electrode potentials are all ca 078 V.
This gives a violet-blue colour in the presence of excess potassium dichromateVI solution. These are diphenylamine diphenylbenzidine and diphenylamine sulfonate. Recommended Videos Acid-Base Titrations.
Then add a few drops of 5 potassium dichromate solution. What is seen by the eye is not the color absorbed but the complementary color from the removal of the absorbed wavelengthsThis spectral perspective was first noted in atomic spectroscopy. The process is called anodizing because the part to be treated forms the anode electrode of an electrolytic cellAnodizing increases resistance to corrosion and wear and provides better adhesion for paint primers and glues than bare metal.
Substances that change their colour when the pH of their surrounding changes are called acid-base indicators. Reaction to form hydrogen chromate ions or dichromate ions affecting the accuracy of the end point. Test for Sulphite ion SO2 3 a On treating sulphite with warm dil.
If no colour change is observed then the alcohol was a tertiary alcohol. Materials Required Apparatus Required weighing bottle weight box volumetric flask conical flask. In the potassium dichromate solution Cr 2 O 7 2-ions in orange colour are in equilibrium with CrO 4 2-ions in yellow colour.
In some reactions the solution changes colour without any added indicator. Gibbs energy change and emf of a cell. Because of the colour change to the acidified potassium dichromateVI solution you must therefore have a secondary alcohol.
Mohrs salt Titration with Potassium Dichromate. H 2 SO 4 SO 2 gas is evolved which is suffocating with the smell of bur ning sulphur. The appearance of the endpoint can be detected by the colour change of KMnO 4 from colourless to light pink.
Potassium nitrite silver nitrite silver salt of fatty acid and lithium-aluminium hydride. Cr 2 O 7 2- H 2 O 2CrO 4 2- 2H Orange-red Yellow. If the potassium dichromate solution changes colour from orange to green then an oxidation reaction has taken place with a primary or secondary alcohol.
Why Does The Colour Change While Potassium Dichromate And Ethyl Alcohol Is Added Quora
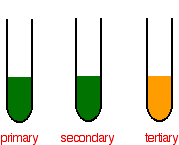
14 7 Determining Alcohol Classifications In The Lab Alternate Reactions Chemistry Libretexts
What Is The Precipitate In Potassium Dichromate Sulfuric Acid And Potassium Iodide Quora

The Colour Change Of Acidified Potassium Dichromate The Real Chemist Youtube
Comments
Post a Comment